Johnson & Johnson pauses coronavirus vaccine trial after participant contracts “unexplained illness”
10/21/2020 / By Franz Walker
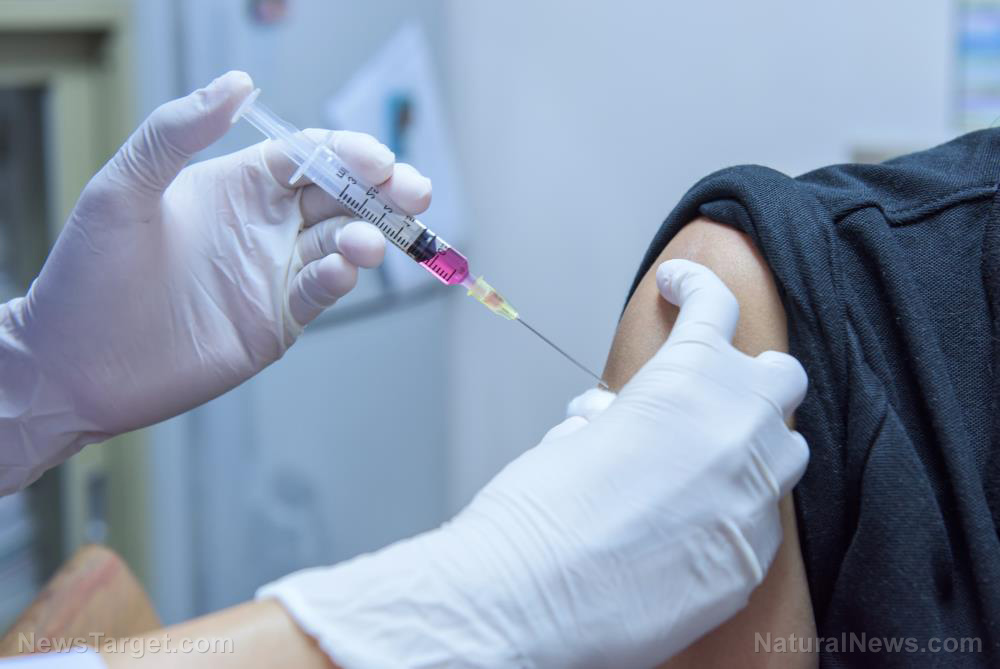
Johnson & Johnson is pausing its Wuhan coronavirus (COVID-19) vaccine trial after a study participant fell ill. The halt comes just weeks after the company announced that they were in the final stage of the trials.
In its news release, Johnson & Johnson said that the trial was paused in compliance with regulatory standards after the unnamed participant developed an “unexplained illness.” It also confirmed that the ENSEMBLE independent Data Safety Monitoring Board (DSMB) reviewed the participant’s condition.
“We must respect this participant’s privacy,” the company’s statement reads. “We’re also learning more about this participant’s illness, and it’s important to have all the facts before we share additional information.”
The statement did not say whether the participant received the experimental vaccine or was in the placebo control group.
NIH says pauses are a good sign
The study pause may be worrying for those concerned about the vaccines’ safety, especially considering the speed at which they’re being developed. National Institutes of Health Director Dr. Francis Collins, however, says that it should actually be reassuring.
According to Collins, the fact that pauses happened means that issues encountered in the testing are not being “glossed over.”
“If anybody thinks we’re just glossing over these kinds of issues in the big rush to approve a vaccine, this ought to be reassuring,” Collins said during an interview with NBC News.
In addition, the 60,000 patient trial is only under a study pause; it is not under what the FDA calls a “clinical hold.” The latter entails a longer delay than a pause, while also placing further limits. These included preventing the recruitment of more participants and taking those already in the study “off therapy involving the investigational drug.”
“A study pause, in which recruitment or dosing is paused by the study sponsor, is a standard component of a clinical trial protocol,” the company said in the statement.
Johnson & Johnson CFO Joseph Wolk added that the company was simply acting responsibly.
“We’re letting safety protocol follow proper procedure here,” he said. “What it should also do is reassure the public that every scientific, medical and ethical standard is being applied here.”
Not the first vaccine test to be paused
Johnson & Johnson is just the latest company to pause its COVID-19 vaccine trial. It joins the likes of AstraZeneca, who paused its own coronavirus vaccine trial last month, right after it started phase 3 trials last month, after a participant in the U.K. reportedly developed neurological symptoms. (Related: Second AstraZeneca trial participant develops rare neurological condition following COVID-19 vaccination.)
AstraZeneca has since resumed its trial with the University of Oxford in the U.K. but has yet to receive approval to do the same in the U.S. from the Food and Drug Administration (FDA).
Experts were split on whether the trials were restarted too quickly, especially with a second volunteer experiencing a neurological event. Some say that more analysis is needed to conclude whether the events are truly independent of the vaccine, while others note that the cases are unrelated to the vaccine’s adenovirus vector and could simply be down to chance.
In July, AstraZeneca CEO Pascal Soriot said that the company had concluded that the neurological symptoms were due to an undiagnosed case of multiple sclerosis, and unrelated to COVID-19. However, experts say that the vaccine candidate needs further investigation because compared to candidates from other companies, AstraZeneca’s adenovirus vector has limited precedence in humans.
The split reflects the differences in decisions from the FDA and regulatory bodies in other regions. The former has broadened its investigation on AstraZeneca’s vaccine candidate while trials remain on hold in the U.S. Regulatory bodies abroad, on the other hand, have allowed trials of the vaccine candidate to continue.
Follow Pandemics.news for more information on the vaccines being tested to fight the coronavirus.
Sources include:
Submit a correction >>
Tagged Under:
AstraZeneca, coronavirus, covid-19, delay, disease, flu, Johnson & Johnson, outbreak, pandemic, pause, side effects, superbugs, vaccine, vaccine injury, vaccine wars, vaccines, virus
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
Trump.News is a fact-based public education website published by Trump News Features, LLC.
All content copyright © 2018 by Trump News Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.






















